Understanding Performance Limiting in Solid-State Batteries
Researchers clarify the role of space-charge layers in limiting the performance of solid-state batteries.
Solid-state batteries (SSBs) are widely considered a promising solution to address the limitations of commercial Li-ion batteries.
Li-ion batteries with liquid electrolytes can leak or catch fire. Image used courtesy of Adobe Stock
Traditional li-ion batteries with liquid electrolytes can leak or catch fire, while SSBs use a solid electrolyte which is much safer and less prone to thermal runaway. SSBs also offer high energy density due to high-energy materials like lithium metal. Furthermore, with such materials, there is no risk of dendrite formation, which can cause short circuits and fires in traditional Li-ion batteries.
Solid-State Battery Challenges and Limitations
However, SSBs have their limitations. One of the critical challenges is the solid-solid interface resistance between the electrodes and the solid electrolyte that can impede the flow of charge carriers and limit the battery's performance. Researchers are exploring strategies to address this challenge by understanding the mechanism behind such significant resistance.
Many researchers refer to the concept of space-charge layers (SCLs) to explain this resistance. A space charge layer is a region of charge imbalance between the solid electrolyte and the electrode. Such an interface usually forms at the interface between two materials with different electronic properties.
This charge imbalance is thought to affect the motion of ions across the interface and the interface resistance. However, the influence of SCLs in limiting battery performance is still unclear.
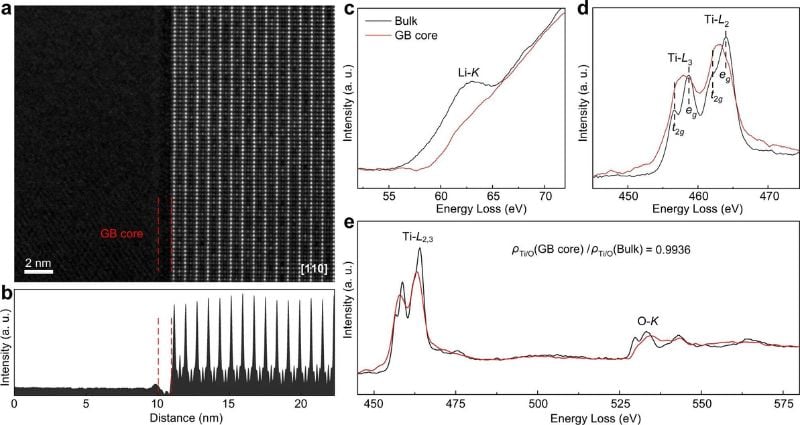
A team of researchers from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) discovered the high resistance in the electrolyte interface is not due to SCLs. Through observations made at the atomic scale, they found that lithium-depleted grain boundary cores cause interface resistance.
New Insights on Solid Electrolyte Interfacial Resistance
If the space charge layer affects the interface resistance, one would expect to observe a Li-deficient region in which the low charge carrier concentration impedes ion transport. To better understand the effect, the team observed the Li0.33La0.56TiO3 material, a solid electrolyte, using atomic-resolution high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM).
Measurements of the charge at the grain-boundary core. Image used courtesy of Nature Communications (2023), DOI: 10.1038/s41467-023-37313-2
The researchers report that this structure leads to quite efficient ion transport. However, they did not observe lithium deficient regions in SCLs; instead, they found that the grain boundary SCLs are Li-excess. They pinpointed the location of the Li-access area in the lattice and then combined theoretical calculations and electrochemical tests to observe its transport properties.
The researchers believe their work will provide insights into optimizing the interface of all solid-state batteries. They also suggest that the knowledge of ion transport in SCLs should not only depend on lithium concentrations and that proper study of atomic configuration is critical.