Using Stress to Control Dendrite Growth in Solid-state Batteries
Massachusetts Institute of Technology (MIT) engineers are paving the road toward a new rechargeable lithium-ion battery that is more lightweight, compact, and safe.
Lithium-ion (Li-ion) batteries are the most popular portable rechargeable battery type today due to their high energy density, power density, and compactness, which other batteries can't offer. Research laboratories worldwide are working on making rechargeable Li-ion batteries more lightweight, compact, and safer.

Massachusetts Institute of Technology. Image used courtesy of Flickr
In a Li-ion battery, lithium ions exchange between two electrodes for energy storage and release. Both anode and cathode contain lithium atoms. During discharging, the anode oxidizes to produce positively charged lithium ions and electrons. Lithium ions move through the electrolyte, and electrons move through the external circuit and recombine at the cathode.
This reaction lowers the chemical potential of the cell and drains the energy from the cell to an external circuit. During charging, the reactions reverse, and electrons go in the opposite direction, increasing the chemical potential of the cell.
The key toward smaller, lighter batteries is replacing the liquid electrolyte with a thinner layer of solid ceramic material and replacing one of the electrodes with solid lithium metal. Solid electrolytes significantly reduce the risk of leaks, a serious safety issue for batteries with liquid electrolytes. Liquid electrolytes are also flammable, increasing the safety risk. The lithium metal electrodes provide excellent electrochemical performance and stability.
However, such solid electrolyte design suffers from dendrite formation. There hasn't been much progress on what causes dendrites to grow and how to prevent them.
Researchers at the Massachusetts Institute of Technology (MIT) seem to have found the answer to the unresolved question of what causes dendrite formation. Moreover, they suggest ways to prevent dendrites from crossing the electrolyte, paving the road for practical, lightweight solid-state batteries.
Dendrite Formation Can Cause Short Circuits
During charging, the lithium metal electrochemically grows irregularly on negative electrodes to form spiky microstructures called dendrites. Dendrite formation is aided by the mass transport of cations surrounded by solvent molecules. The solvated cations shed the solvent molecules and reduce to absorb atoms that diffuse on the surface and get incorporated by metal lattice.

Image used courtesy of Adobe Stock
Dendrites can cause short circuits and lead to catastrophic failures in the long term. They can build on lithium surfaces and penetrate through solid electrolytes, shorting the cell. Other effects of dendrite formation can affect battery performance. The dendrites react with the electrolyte and decompose it, triggering the loss of active lithium inside the battery and causing capacity loss.
MIT Researchers Study Lithium Dendrite Formation
In their paper published in the journal Joule, the researchers report that soft lithium metal can penetrate solid electrolyte material during the charging and discharging of a battery as lithium ions move between the two electrodes. This movement causes the volume of electrodes to change, stressing the solid electrolyte.
The electrolyte has to remain completely in contact with both electrodes, and even microscopic flaws can create pressure that causes cracking. These cracks allow dendrites to form.
To observe dendrite formation, MIT graduate student Cole Fincher developed a way of making thin cells using a transparent electrolyte. Researchers wanted to see whether the dendrites behave similarly to a corrosion or fracture process.
Controlling Dendrite Growth by Applying Stress
The team shows manipulation of dendrite growth by applying and releasing pressure, causing the dendrites to propagate (zig and zag) in alignment with the direction of force and parallel to two electrodes. Researchers say there are other ways to exert the needed stress, including thermal expansion. The electrolyte can include two layers of materials with different amounts of thermal expansion, creating an inherent bending of the material.
Another way could be doping the material with embedded atoms for a permanently stressed state. The researchers found that the amount of pressure needed is not extreme. Their experiments showed that a pressure of 150 to 200 megapascals was sufficient to stop the dendrites from crossing the electrolyte.
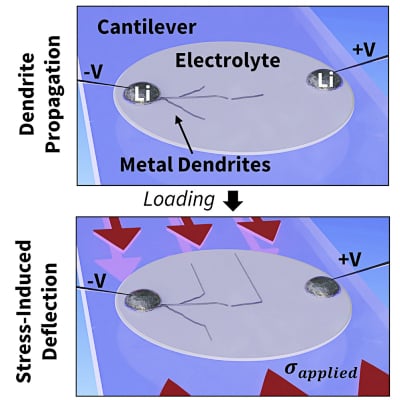
Illustration of controlled dendrite growth. Image used courtesy of MIT
Researchers believe their study could make it practical to produce batteries using solid electrolyte and metallic lithium electrodes. Now they plan to apply these basic principles to a prototype battery and find the manufacturing processes needed to produce such solid-state batteries in quantity.






