Research: Electrochemical Mechanisms for Enhanced Energy Storage
Researchers from Drexel University have developed a technique that combines ultraviolet-visible spectroscopy and cyclic voltammetry to rapidly identify the precise electrochemical mechanisms involved in batteries and supercapacitors of different materials.
Electrochemical energy storage is a critical technology that enables access to clean, reliable, and sustainable energy.
Renewable energy: wind, solar, biomass, hydropower. Image used courtesy of Adobe Stock
Advancing the benefits of electrochemical energy storage requires understanding why some materials outperform others in terms of energy storage. Researchers from Drexel University have developed a new technique that rapidly identifies the precise electrochemical mechanisms involved in batteries and supercapacitors of different materials.
This research breakthrough has the potential to significantly enhance the performance of energy storage devices, subsequently improving the capacity of portable electronic devices, electric vehicles, and renewable energy grids.
Observing Electrochemical Systems
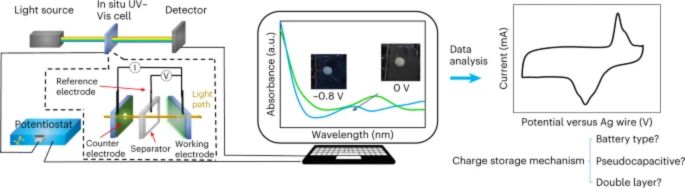
The Drexel team’s technique, published in Nature Energy, merges two well-known scientific research approaches. The first involves determining the composition of chemical compounds based on their visible light absorption, while the second measures the electrical current in energy storage devices such as batteries and supercapacitors.
The setup for in situ electrochemical UV-Vis spectroscopy. Image used courtesy of Nature Energy
These two tests, when run simultaneously, give the researchers a more accurate method of monitoring the movement of ions within the devices. This enables them to uncover the complex electrochemical process governing usable power creation.
Despite being a long-time and well-studied field, accurately measuring and monitoring intricate electrochemical systems while they are in operation is challenging and continues to be researched. This has made it difficult to fully understand the role of electrochemical processes in energy storage systems.
The primary obstacle in observing these electrochemical systems is that ions‒the charged atomic particles that generate an electric current when they move within a device‒are not visible. They are too small and move too quickly to be directly observed.
Therefore, researchers must rely on signals that indicate their probable presence, similar to low-resolution atomic radar, by firing particles at them and recording the resulting reflections.
Since it is not possible to see how ions are arranging themselves within, on top of, and between the energy storage compartments known as electrodes, it can be difficult to design them in a way that maximizes energy storage capacity and allows for the ions to enter and exit the device in an orderly manner.
The researchers see their new method as a solution to designing a structure that can better accommodate the ions within an energy storage device to increase efficiency.
Ion Arrangement
How ions are arranged in batteries or supercapacitors is important for their overall performance, including their energy density, power density, life cycle, and safety.
When ions are stored in an electrode, they can assemble in three different ways: within the atomic layers of the electrode material, on its surface, or atop other ions already on the surface. Each of these arrangements affects battery or supercapacitor performance differently.
When ions enter the atomic layers of the electrode material‒intercalation‒more ions can assemble and store more energy within the electrode. Ions that attach or detach to the surface of the electrode material‒surface redox reaction‒enable a quick release of energy. Ions that collect on top of other ions‒an electrical double-layer reaction‒allow for a slightly larger power discharge but less energy than intercalation.
Observing and comparing ion arrangement within an electrode and how long a storage device takes to discharge and recharge can help researchers understand the underlying reactions. Distinguishing the reactions will help improve the efficiency of energy storage devices.
Ultraviolet-visible Spectroscopy
Ultraviolet-visible (UV-vis) spectroscopy is a technique used to analyze the absorption or transmission of ultraviolet and visible light by a sample.
Cyclic voltammetry (CV) is a versatile technique that can be used to study the thermodynamics and kinetics of electrochemical reactions, characterize electrode materials, and investigate electrochemical mechanisms.
The Drexel team’s method combines UV-vis spectroscopy and CV to identify the occurrence of a reaction and the number of electrons being transferred during the reaction. This approach helps to determine the specific type of electrical mechanism taking place.
UV-vis spectroscopy was used to observe electrochemical interactions in thin nanomaterial films of electrode-electrolyte systems. This approach was unconventional but effective because the transparency of the thin electrode material allowed for UV-vis spectroscopy to characterize its electrochemical changes. The researchers managed to eliminate the uncertainty surrounding the electrochemical behavior they were studying by synchronizing the UV-vis spectral data with the CV measurements of current.
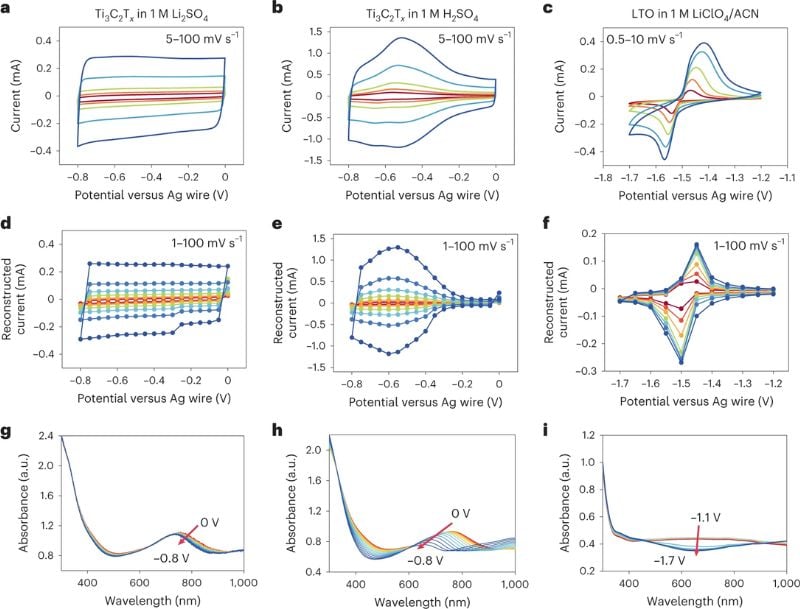
Electrochemical CV curves, reconstructed CV curves, and in situ electrochemical UV-Vis spectra. Image used courtesy of Nature Energy
The researchers plotted UV-vis data on a graph with CV measurements to create a "UV-vis CV" curve, identifying different electrochemical mechanisms by their distinctive curves. The shape of the curve indicates the type of reaction occurring, such as electrical double-layer charging or redox reactions.
This method allowed the researchers to quantify the number of electrons transferred during the reaction and differentiate between electrical double-layer, pseudocapacitive, and intercalation-based battery-type redox processes. They found that this approach is similar to in situ synchrotron X-ray absorption spectroscopy.
System Application
The correlation between UV-vis data and CV measurements allowed the team to obtain more precise measurements of how the electrode material’s electron structure changes during charge-discharge cycling.
This method was more accurate than current methods like X-ray absorption or electron energy loss spectroscopy, which are more expensive and time-consuming. Cross-referencing these measurements can also eliminate the effects of parasitic reactions and further improve the accuracy of the results.
While the method's current application is limited to transparent electrode materials, it could spur materials development for energy storage, capacitive water deionization, electrochemical actuation, and energy harvesting. The team plans to continue its work by testing new combinations of electrolyte and electrode materials and investigating more complex electrochemical energy storage systems.