Mg-Metal Batteries will Nearly Double the Energy Density of Li-Ions
Scientists at the Department of Energy's National Renewable Energy Laboratory (NREL) have discovered a new approach for developing a rechargeable non-aqueous magnesium-metal battery.
A proof-of-concept paper published in Nature Chemistry detailed how the scientists pioneered a method to enable the reversible chemistry of magnesium (Mg) metal in the noncorrosive carbonate-based electrolytes and tested the concept in a prototype cell. The technology possesses potential advantages over lithium-ion batteries—notably, higher energy density, greater stability, and lower cost.
"Being scientists, we're always thinking: what's next?" said Chunmei Ban, a scientist in NREL's Materials Science department and corresponding author of the paper, "An Artificial Interphase Enables Reversible Magnesium Chemistry in Carbonate Electrolytes." The dominant lithium-ion battery technology is approaching the maximum amount of energy that can be stored per volume, she said, so "there is an urgent need to explore new battery chemistries" that can provide more energy at a lower cost.
"This finding will provide a new avenue for magnesium battery design," said Seoung-Bum Son, a former NREL postdoc and scientist at NREL and first author of the paper. Other co-authors from NREL are Steve Harvey, Adam Stokes, and Andrew Norman.
 NREL researchers (from left) Seoung-Bum Son, Steve Harvey, Andrew Norman and Chunmei Ban are co-authors of the Nature Chemistry white paper, "An Artificial Interphase Enables Reversible Magnesium Chemistry in Carbonate Electrolytes" working with a Time-of-flight secondary ion mass spectrometry. The device allows them to investigate material degradation and failure mechanisms at the micro- to nano-scale. (Photo by Dennis Schroeder / NREL)
NREL researchers (from left) Seoung-Bum Son, Steve Harvey, Andrew Norman and Chunmei Ban are co-authors of the Nature Chemistry white paper, "An Artificial Interphase Enables Reversible Magnesium Chemistry in Carbonate Electrolytes" working with a Time-of-flight secondary ion mass spectrometry. The device allows them to investigate material degradation and failure mechanisms at the micro- to nano-scale. (Photo by Dennis Schroeder / NREL)
An electrochemical reaction powers a battery as ions flow through a liquid (electrolyte) from the negative electrode (cathode) to the positive electrode (anode). For batteries using lithium, the electrolyte is a salt solution containing lithium ions. What's also important, is the chemical reaction must be reversible so that the battery can be recharged.
Magnesium batteries theoretically contain almost twice as much energy per volume as lithium-ion batteries. But previous research encountered an obstacle: chemical reactions of the conventional carbonate electrolyte created a barrier on the surface of magnesium that prevented the battery from recharging. The magnesium ions could flow in a reverse direction through a highly corrosive liquid electrolyte, but that barred the possibility of a successful high-voltage magnesium battery.
In seeking to overcome these roadblocks, the researchers developed an artificial solid-electrolyte interphase from polyacrylonitrile and magnesium-ion salt that protected the surface of the magnesium anode. This protected anode demonstrated markedly improved performance.
The scientists assembled prototype cells to prove the robustness of the artificial interphase and found promising results: the cell with the protected anode enabled reversible Mg chemistry in carbonate electrolyte, which has never been demonstrated before. The cell with this protected Mg anode also delivered more energy than the prototype without the protection and continued to do so during repeated cycles.
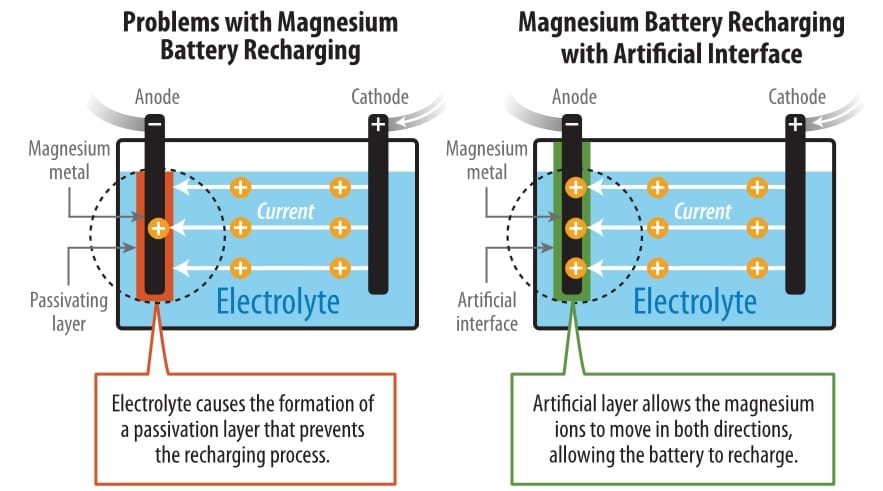 Illustration shows how NREL researchers have addressed the problem with making a rechargeable magnesium battery. (Illustration by John Frenzl / NREL, click on image to enlarge)
Illustration shows how NREL researchers have addressed the problem with making a rechargeable magnesium battery. (Illustration by John Frenzl / NREL, click on image to enlarge)
Furthermore, the group has demonstrated the rechargeability of the magnesium-metal battery, which provides an unprecedented avenue for simultaneously addressing the anode/electrolyte incompatibility and the limitations on ions leaving the cathode.
In addition to being more readily available than lithium, magnesium has other potential advantages over the more established battery technology. First, magnesium releases two electrons to lithium's one, thus giving it the potential to deliver nearly twice as much energy as lithium.
And second, magnesium-metal batteries do not experience the growth of dendrites, which are crystals that can cause short circuits and consequently dangerous overheating and even fire, making potential magnesium batteries much safer than lithium-ion batteries.
Other researchers involved with the project were Tao Gao and Chunsheng Wang of the University of Maryland; K. Xerxes Steirer of Colorado School of Mines; and Arthur Cresce and Kang Xu of the U.S. Army Research Laboratory.
Funding for the research came from the Laboratory Directed Research and Development Program at NREL.
NREL is the U.S. Department of Energy's primary national laboratory for renewable energy and energy efficiency research and development. NREL is operated for the Energy Department by The Alliance for Sustainable Energy, LLC.






