Tackling EV Range Anxiety With Faster-Charging Indium-Anode Chemistries
A research team at Cornell University is developing faster-charging battery chemistries to combat electric vehicle range anxiety.
One challenge facing the widespread adoption of electric vehicles (EVs) is range anxiety, the fear of an EV running out of charge during a trip. Limited by the charging rates of conventional lithium batteries, the industry is now turning to novel battery chemistries to achieve faster charging rates.
Researchers from Cornell University have developed a battery capable of fully charging in under five minutes. This article will explore the inherent limitations causing lithium batteries to charge slowly and how the Cornell team’s battery solves these challenges.
Electric vehicle low battery. Image used courtesy of Adobe Stock
What Influences Battery Charging Speed?
Slow charging rates are a major concern for EV owners. But what are the factors influencing the speed of lithium battery charging?
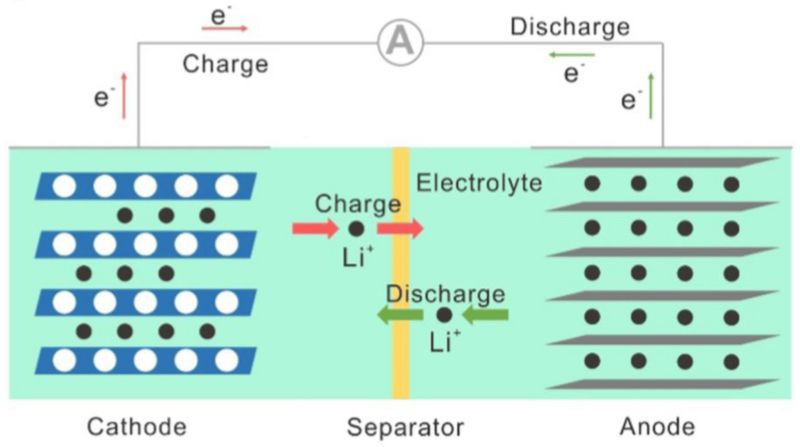
One key factor is the lithium-ion diffusion rate within the battery's electrodes. In the charging process, the lithium ions must move through the electrolyte and into the electrode's structure, a process limited by the physical and chemical properties of the electrode materials. For instance, the microscopic structure of the electrodes can either facilitate or impede the movement of lithium ions. Materials with a porous or layered structure can offer more pathways for ion movement, enhancing diffusion rates.
Lithium-ion battery working principle. Image used courtesy of Wang et al.
However, the chemical composition and bonding within the electrode material also affect how readily lithium ions can be inserted or removed. Stronger chemical bonds or interactions with the host material might slow the ion movement, leading to slower charging times. This intricate interplay between the material's structure and chemistry is a key research area in battery technology. Scientists aim to find the optimal balance, allowing rapid ion diffusion without compromising the battery's stability or capacity.
Heat generation is another critical factor. As the battery charges, it generates heat, leading to thermal management problems. If the temperature rises too high, it can slow the charging process or even halt it to prevent damage. This is particularly challenging in rapid charging scenarios, where high currents cause increased heat generation.
Cornell’s Lithium Battery Research
To target the issue of range anxiety in electric vehicles, researchers from Cornell University recently developed a lithium battery capable of charging in under five minutes, a significant advancement over existing technologies.
The research team developed a lithium battery with indium as the anode. Image used courtesy of Jin et al.
To accomplish this, the researchers focused on the kinetics of electrochemical reactions, particularly the concept of the Damköhler number, which balances the rate of chemical reactions with the transport rate of materials to the reaction site. This approach led them to develop a battery with indium as the anode material for their battery. According to the researchers, indium is notable for its low migration energy barrier, facilitating rapid ion diffusion, and its modest exchange current density, which is linked to the anode's ion reduction rate. This combination of fast diffusion and slow surface reaction kinetics is crucial for achieving rapid charging and prolonged storage.
The team’s innovation lies in the electrode design, which ensures metal ions at the battery anode move freely, allowing for stable electrode morphology in each charging cycle. This design principle significantly enhances the battery's ability to charge and discharge repeatedly over thousands of cycles.
Future Research in EV Batteries
The research team notes indium is promising but has limitations, such as its weight, which poses challenges for practical applications. The team recommends further research, possibly using computational chemistry and artificial intelligence tools, to find lightweight materials that perform similarly.