Major Developments in Battery Technology, Materials, Research
Four recent developments in battery technology could lead to improved performance and range in electric vehicles. This article reviews those advances and explains how each contributes uniquely to the evolution of battery technology.
As consumers demand electric vehicles (EVs) with longer ranges, lower costs, and more reliability, researchers have been working hard to explore ways to improve EV battery pack performance.
Ford Lightning battery pack. Image used courtesy of Ford
The demand for better battery packs has led to rapid changes in battery design, with the industry desperately aiming for enhanced performance, sustainability, and safety. Four studies have developed materials and technologies that could lead to major EV battery and energy storage advancements.
Xanthan Gum in Battery Protection
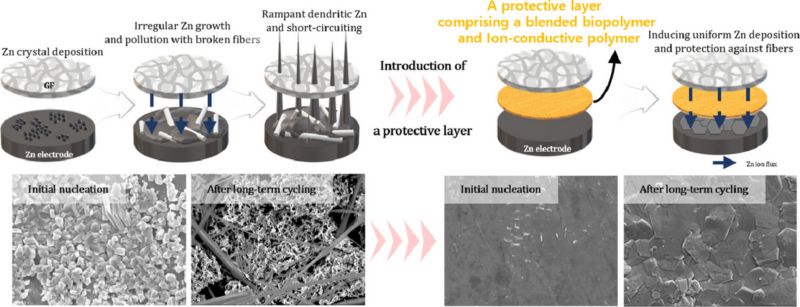
Researchers at Pohang University of Science and Technology have discovered a novel use for xanthan gum, a plant-derived biopolymer commonly used in cosmetics. With the biopolymer, they've created a protective film for battery electrodes by blending xanthan gum with an ionically conductive polymer. This film significantly improves the durability of electrodes in energy storage systems, crucial for harnessing renewable energy like solar power.
The researchers use xanthan gum to create a protective battery layer. Image used courtesy of Jang et al.
One key research focus is zinc-ion batteries, a promising alternative to lithium-ion batteries. These batteries are safer in terms of fire risks and can store substantial amounts of energy. However, they face challenges such as the formation of twig-like crystals on the zinc surface during charging and discharging cycles, reducing battery longevity. The protective film developed addresses this issue by facilitating uniform zinc nucleation and preventing crystal formation, thus enhancing battery life and stability.
U.S. Investment in Battery Industry
The U.S. Department of Energy recently announced an investment of $3.5 billion to strengthen the domestic battery supply chain. The initiative aims to secure a domestic supply chain and reduce dependence on international sources, particularly in light of the growing demand for lithium batteries, projected to increase tenfold by 2030.
This funding, part of the Bipartisan Infrastructure Law, targets companies producing batteries and the critical minerals necessary for them. This underscores the critical role of batteries in addressing climate change, as they are essential for electric vehicles and storing electricity from renewable sources. Additionally, the funding will support companies working on alternative battery chemistries, such as flow and sodium batteries, reflecting a broadening scope in battery technology research and development.
Cooperative Ion Behavior in Batteries
Scientists at the U.S. Department of Energy's Argonne National Laboratory have recently discovered electrolyte behavior within batteries.
The researchers investigated the combination of different anion and cation types. Image used courtesy of Driscoll et al.
They found that combining different types of anions with cations can markedly improve battery performance. This finding is particularly relevant for multivalent batteries, which use cations like zinc, magnesium, and calcium with a +2 charge, as opposed to lithium's +1 charge. These batteries could offer higher energy storage and release, making them suitable for electric vehicles and grid storage.
The research revealed that specific anions can influence the metal deposition rate and stripping rate at the battery's anode, crucial for efficient energy conversion. This insight opens up new possibilities for designing advanced battery electrolytes by fine-tuning ion interactions to optimize performance.
Shaping Hard Carbon Electrodes
Another recent study from the Tokyo University of Science, Japan, has made significant strides in developing advanced electrode materials for sodium (Na)-ion batteries (NIBs) and potassium-ion batteries (KIBs). This research focuses on nanostructured "hard carbon" (HC) electrodes. Unlike other forms of carbon, HC is amorphous, lacking a well-defined crystalline structure, yet it is robust and resistant.
Nanopores enable materials to store more charge carriers. Image used courtesy of Igarashi et al.
In an innovative approach, the team used zinc oxide (ZnO) as a template during HC synthesis, creating nanopores that enhance the material's capacity to store charge carriers. This technique has shown considerable promise, especially for the negative electrode of NIBs, with the optimized ZnO-templated HC demonstrating a reversible capacity of 464 mAh g–1 and a high initial Coulombic efficiency of 91.7%. Remarkably, the energy density achieved in NIBs using this material was 312 Wh kg–1, comparable to certain commercial lithium-ion batteries and significantly surpassing the energy density of earlier NIBs.
The study's findings underscore the potential of using inorganic nanoparticles as templates to control pore structure in developing HC electrodes. This advancement positions HCs as potential candidates for negative electrodes, offering a sustainable alternative to graphite. Such development is pivotal for applying NIBs in consumer electronics, electric vehicles, and low carbon footprint energy storage systems, especially for storing energy from renewable sources like solar and wind farms.