Researchers Turn to Toothpaste for Improved Battery Life
Adding a fluorinated cation could introduce greater battery health and longer lifetimes.
Lithium-metal batteries are considered a promising alternative to lithium-ion batteries owing to their greater theoretical energy capacity. Unfortunately, in practice, lithium-metal batteries are limited in performance and safety by unwanted dendrite growth due to SEI layer formation.
An ingredient in toothpaste could improve battery life. Image used courtesy of Pexels
Recently, researchers from Argonne National Laboratory addressed the issue of dendrite formation in lithium-metal batteries.
This article will discuss lithium-ion interphases, challenges with lithium-metal batteries, and new research.
Lithium Ion Interphases
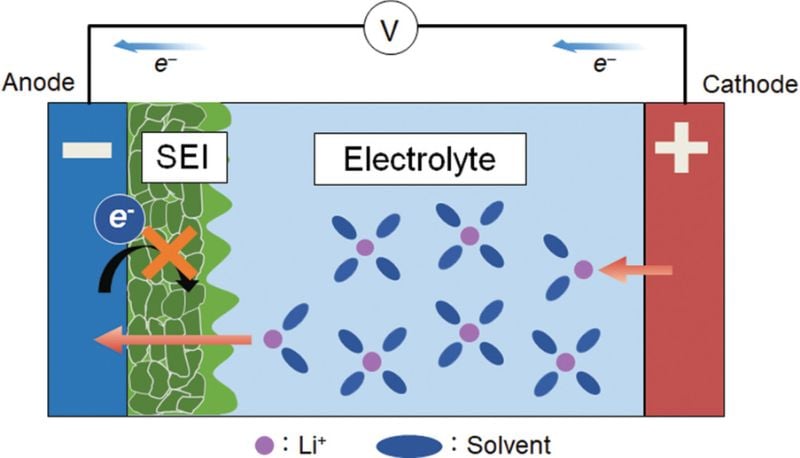
In lithium-ion batteries, the solid electrolyte interphase (SEI) is a thin layer that forms on the electrode surface during the first charging cycle of the battery. This layer is a product of the decomposition of electrolyte components, which occurs when the electrode potential falls below a certain threshold. In general, the SEI layer is primarily composed of lithium salts, organic polymers, and inorganic compounds such as lithium oxide and lithium fluoride.
The formation of an SEI layer in a lithium-ion battery. Image used courtesy of Takenaka et al.
The SEI layer serves several critical functions in a lithium-ion battery. First, it acts as a physical barrier to prevent contact between the electrolyte and the electrode. This is an important safety feature as it prevents short circuits and side reactions that can degrade the battery's performance over time. Second, it is electronically insulating but ionically conductive, allowing lithium ions to pass through while blocking electrons, which is essential for the battery's operation.
However, the formation and evolution of the SEI layer can also lead to capacity loss and aging in lithium-ion batteries. The layer's growth consumes lithium ions, reducing the number of ions available for the main charge-discharge reactions and decreasing the battery's capacity. Furthermore, an unstable SEI layer can lead to the formation of lithium dendrites, which can cause short circuits and pose safety risks.
Lithium-Metal Challenges
While the challenges surrounding the SEI layer are manageable in lithium-ion batteries, in lithium-metal batteries, they are a limiting factor.
The major issue with lithium-metal batteries is that their high energy density declines rapidly with repeated charging and discharging. Due to the chemistry of lithium-metal batteries, the electrolyte generally does not form an adequate SEI layer during the initial cycles, which leads to lithium dendrites. The formation of these dendrites not only poses safety risks in lithium-metal batteries but also reduces the number of ions available for the main charge-discharge reactions, decreasing the battery's capacity.
For lithium-metal batteries, which are meant to have a greater capacity than lithium-ion batteries, the performance loss due to dendrite formation is a significant drawback and limits commercial viability.
New Research on Dendrite Formation in Lithium-Metal Batteries
Researchers from the Argonne National Laboratory recently found a solution that could solve dendrite formation in lithium-metal batteries.
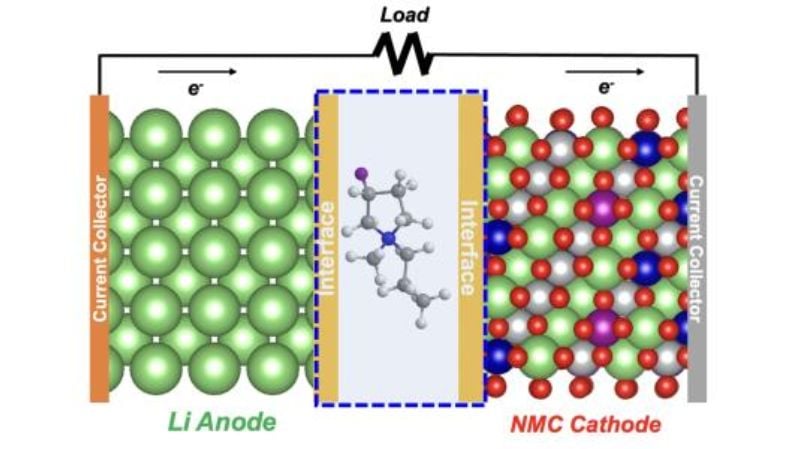
The team’s solution involved introducing an ionic liquid that couples a fluorinated component that is positively charged (cation) with a different fluorinated component that is negatively charged (anion) into the battery’s electrolyte. By using the computing resources of the Argonne Leadership Computing Facility (ALCF), simulations of the new electrolyte revealed that the fluorine cations stick to and accumulate on the anode and cathode surfaces of the lithium-metal batteries before any charge-discharge cycling and before the formation of an SEI layer. The accumulation of the fluorine cations ultimately acted as a form of protection for the anode and cathode surfaces and helped to mitigate the impact of dendrite formations.
A lithium-metal battery with a fluorinated cation. Image used courtesy of Argonne National Laboratory
The team was able to tune the proportion of fluoride solvent to lithium salt to create a layer with optimal properties, such as the thickness of the SEI layer. Because of this layer, lithium ions could efficiently flow in and out of the electrodes during charge and discharge for hundreds of cycles.