Problem Solved? Solid Electrolyte Balances Enhanced Performance With Safety
The University of Liverpool reveals a groundbreaking solid-electrolyte material that conducts lithium ions at speeds matching liquid electrolytes.
While ongoing innovation in battery performance is crucial for advancement, safety considerations must remain paramount. Recently, the University of Liverpool made a groundbreaking discovery of a solid electrolyte capable of delivering enhanced performance while maintaining impeccable safety standards.
The research addresses the tradeoff between battery performance and safety. This article examines these tradeoffs and explains how Liverpool’s research aims to address this conflict.
University of Liverpool materials laboratory. Image used courtesy of University of Liverpool
The Battery Safety Dilemma Explained
Within a standard lithium-ion battery, the electrolyte serves as a medium for transporting ions between the electrodes, facilitating the flow of electric current within the cell. Chemical energy converts to electrical energy during the charging cycle, and the reverse happens during the discharging cycle. This process powers the battery.
Two major types of electrolytes are used today: liquid and solid.
The fluid nature of liquid electrolytes provides numerous pathways for lithium ions to navigate, whereas pathways are more limited in solid electrolytes. As a result, liquid electrolytes tend to offer higher ionic conductivity than solid electrolytes, but at the expense of additional safety measures due to potential leakage, flammability, and explosion.
Dendrite formation on lithium. Image used courtesy of MIT (by Peng Bai)
In contrast, solid electrolytes provide enhanced safety by reducing the chances of explosion and inhibiting dendrite formation. Because of this greater inherent safety, solid electrolytes enable unprecedented features like metallic lithium anodes, thinner battery designs, higher energy density, higher safety, and improved stability. Still, historically, solid electrolytes have suffered from lower ionic conductivity than liquid electrolytes and more challenges in manufacturing.
Making the right trade-off between performance and safety is essential to optimizing battery design according to requirements. Battery performance and safety can be optimized individually at their maximum levels, but achieving peak optimization for both simultaneously has remained a notable challenge so far.
Solid Electrolyte Offers Solid Performance and Safety
University of Liverpool researchers recently developed a solid electrolyte that delivers performance equivalent to liquid electrolytes while significantly enhancing safety.
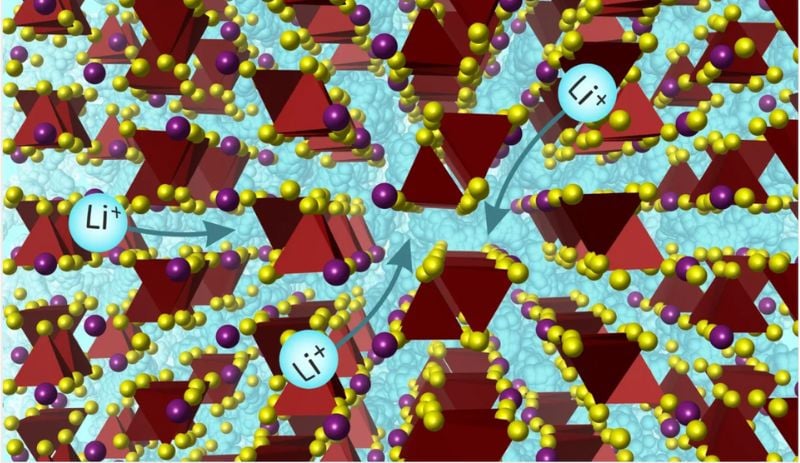
The team tackled the ion-transport challenge by introducing a fresh solid electrolyte comprising lithium, silicon, sulfur, and iodine. This material boasts a unique three-dimensional crystal structure, offering an impressive power density and 15 pathways for lithium-ion movement. This structural diversity facilitates efficient ion conductivity, minimizing the likelihood of ion trapping and improving overall battery performance. Moreover, it demonstrates a low activation energy, indicating minimal energy barriers for lithium-ion movement within the crystal lattice.
The researchers confirmed the material's stability and performance through various destructive experiments, including short circuit tests, nail penetration tests, heating tests, and overcharge tests. Unlike liquid electrolytes, which may leak or explode under such conditions, the solid-state electrolyte exhibited high safety performance.
Lithium ions pathways in solid electrolyte. Image courtesy of University of Liverpool
During the research and development phase, artificial intelligence helped pinpoint favorable chemical compositions, directing researchers toward materials possessing distinctive structural features conducive to ion transportation.
To test the electrolyte, the team devices an all-solid-state cell setup with a lithium cobalt oxide cathode and lithium metal anode. The material exhibited superior energy density, power density, and cycle life compared to conventional liquid electrolytes.
Future Solid-State Prospects
Rather than competing with current solid-state technologies, this research marks a new direction in changing the battery landscape. However, it is not without challenges.
The team notes the material's susceptibility to air and moisture poses a considerable impediment in manufacturing, mandating controlled environments during battery assembly to ensure stability. While the material demonstrates promising performance characteristics, further investigation is needed to enhance its synthesis, scalability, and alignment with existing battery technologies.