Sweet Solution: Chocolate Truffle-Inspired Battery Tackles EV Dendrite Formation
A lithium metal battery design from Harvard University promises significant advancements for electric vehicle charging.
Researchers have developed a solid-state battery design they compare to a chocolate truffle. The unique design of their lithium metal batteries addresses a nagging problem in electric vehicle (EV) batteries: the formation of dendrites.
These small, tree-like structures accumulate on the surface of a lithium metal anode during charge and discharge cycles. They can cause short circuits and weaken cell performance.
EV charging. Image used courtesy of Pexels/by Kindel Media
To control dendrite formation, scientists from Harvard University’s School of Engineering and Applied Sciences (SEAS) devised a chocolate-like design that wraps the lithium metal solid-state battery anode in a protective coating. Xin Li, who co-authored the study, likens the concept to a hard shell encasing a hazelnut core inside a chocolate truffle.
The postage stamp-sized pouch cell features a lithium metal anode, offering 10 times the capacity of existing graphite anodes. This selection brings the potential to increase driving range.
The breakthrough unlocked substantial technical improvements. The battery retained 80% of its capacity after 6,000 charge and discharge cycles, beating the performance of existing pouch cell batteries. It can also be charged in about 10 minutes.
Dendrite Formation in Solid-State Batteries
In designing solid-state lithium metal batteries, it’s important to limit or eliminate the formation of dendrites on the anode’s surface. The phenomenon occurs after the ions move from the cathode to the anode when charging and attach to the anode’s surface via plating. This creates an uneven surface with the ideal conditions for dendrite growth. Irregular plating slows the stripping sequence upon discharging, where the coating is pulled away from the anode. As a result, the surface wears down even more in future charging cycles.
Dendrites could grow into the electrolyte and interfere with the separator material between the cathode and anode, allowing an internal short in the battery. In addition to catastrophic failures and short circuits, the presence of dendrites also reduces performance and battery life.
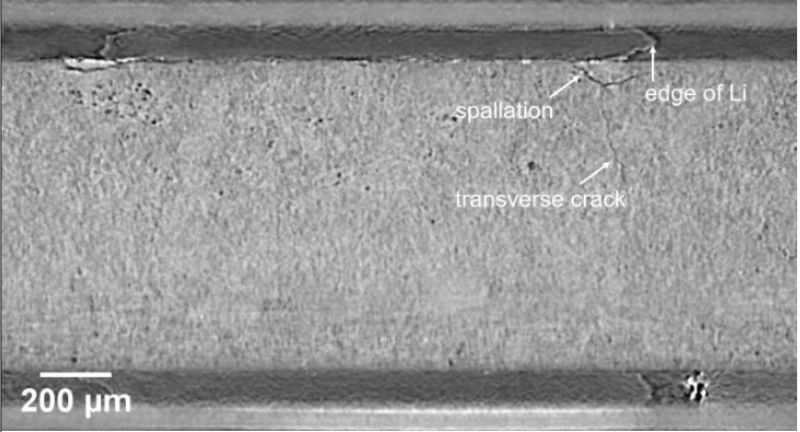
Researchers from the University of Oxford studied dendrite formation, which can later cause cracks in the electrolyte after subsequent charging cycles. They found that above the critical charge density, pothole-like cracks formed on the electrolyte’s surface. Then, subsequent charging cycles showed lithium growing deeper into the cracks, which could induce short circuits.
X-ray computed tomography reveals the spallation and cracking effects of dendrites forming in a solid-state cell while charging. Image used courtesy of the University of Oxford/by Ziyang Ning
Harvard Researchers’ Solution to Dendrites
The Harvard SEAS team has been researching the dendrite phenomenon for years, and the latest battery design expands upon their previous studies. In a 2021 paper, they designed a multilayer battery that placed materials with varying stabilities between the anode and cathode. They started with a control-based approach to limiting lithium dendrite formation, devising a design preventing penetration into the materials. Using an electrolyte immune to dendrites but less stable with lithium could limit dendrite growth to the graphite layer and first electrolyte with no further expansion. Controlling the mechano-electrochemical environment enhanced the stability of the solid-electrolyte and battery system.
A visualization from 2021 shows the first electrolyte (in green) susceptible to dendrite formation but stable with lithium. In the second, less stable electrolyte (brown) dendrites grew into the graphite and the first electrolyte but stopped there. Image used courtesy of Harvard SEAS/by Second Bay Studios
Their latest research combating dendrites uses micrometer-sized silicon particles in the anode, allowing researchers to constrict the lithiation reaction so ions only attach to the surface of the silicon particle but stop there.
The coated particles create an even surface where the current density can be evenly distributed. This speeds up battery recharging to 10 minutes because the even surface accelerates the plating and stripping process. Like the chocolate shell of a truffle, the design wraps lithium metal around the silicon particle, acting as the core.
The Harvard researchers said they focused on silicon, a well-established anode material, to demonstrate that the lithiation reaction of micron-sized silicon could be constricted at the solid-solid interface. That way, the reaction occurs only at thin surface sites of silicon particles due to a diffusion-limiting process. This differs from the conventional lithium-silicon alloying in solid-liquid designs. In liquid lithium-ion batteries, silicon particles are destroyed in the anode through a lithiation reaction.
Harvard’s Office of Technology Development licensed the technology to Adden Energy, a university spinoff that Li co-founded. The Massachusetts-based company has increased the pouch cell to a smartphone size and plans to scale the battery’s performance into commercial Amp-hour-sized cells.