Exploration of Innovative Lithium-Ion Conductor Redefines Battery Sustainability
The University of Liverpool develops a novel solid electrolyte capable of overtaking liquid electrolytes in performance and safety.
Batteries are the silent heroes, powering everything from the smallest gadgets to the largest endeavors. Lithium-ion batteries have become a standard solution, but they often fall short of meeting power, safety, and longevity needs.
University of Liverpool researchers unveiled a solid electrolyte with potential to address the global demand for carbon-free electrification without the problems lithium-ion batteries pose. But what exactly are the limitations of traditional battery technology, and how does the Liverpool research aim to address these shortcomings?
Lithium-ion battery. Image used courtesy of Adobe Stock
Lithium Ion Batteries and Their Challenges
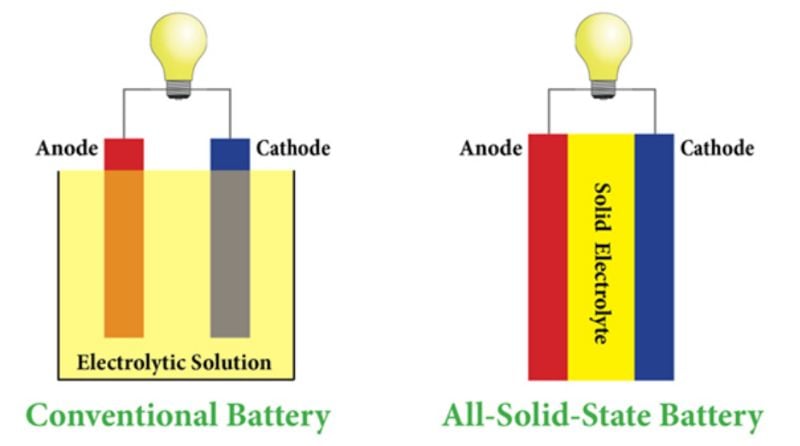
A traditional lithium-ion battery predominantly features a cathode submerged within a liquid electrolyte solution, separated by a discerning membrane, and a lithium-based anode. During discharge, electrical energy is generated by lithium ions moving from anode to cathode through the electrolyte. Recharging the battery reverses this process, allowing lithium ions to return to the anode.
Despite their widespread use, conventional lithium-ion batteries face challenges such as limited energy density, potential safety concerns, and resource constraints. In many ways, the electrode conductivity significantly influences lithium-ion batteries' effectiveness. As a result, researchers devote substantial resources to refining the electrochemical properties of electrodes by integrating diverse, innovative materials into their design and application.
Model of liquid and solid electrolytes. Image used courtesy of Department of Energy
Solid electrolytes are non-liquid materials that conduct ions in a battery. They offer advantages such as improved safety due to reduced risk of leakage or combustion, potentially allowing the use of metallic lithium anodes and higher energy density. However, solid electrolytes may have lower ionic conductivity compared to liquid electrolytes, which can affect battery performance. Liquid electrolytes, on the other hand, are typically organic solvents containing lithium salts, offering high ionic conductivity but posing safety risks such as flammability and leakage.
Adopting a solid electrolyte that matches the performance of liquid electrolytes while enhancing safety could address many challenges in the battery industry.
Solid Electrolyte With Fast Conductivity
In the University of Liverpool’s study, the team discovered a solid material that quickly conducts lithium ions.
In typical solid electrolytes, the conduction pathways exhibit a singular coordination geometry. Instead, the researchers manufactured electrolytes utilizing a Li7Si2S7I chemistry featuring ion arrangements akin to those found in intermetallic systems. This arrangement resulted in anion packing alternating between hexagonal close-packed structures. It sheared face-centered cubic-like motifs to accommodate sulfur and iodine complexes, similar to the nickel-zirconium structure.
Consequently, the materials feature a network of 15 distinct lithium sites with varied geometries and anion coordination. This diverse array of conduction pathways facilitates high conductivity for lithium ions.
The team arrived at their final result thanks to a collaborative interdisciplinary research effort around synthesizing the material, its structure, and validation in battery cells. Leveraging computational modeling and experimental techniques, researchers combined artificial intelligence and physics-based calculations to guide decision-making throughout the discovery process. This approach enabled the identification of a material structure redefining the concept of high-performance solid-state electrolytes.
The material, composed of non-toxic and abundant Earth elements, exhibits superior conductivity to conventional solid-state electrolytes, potentially allowing it to replace liquid electrolytes without compromising battery performance. Its high lithium ion conductivity enhances battery safety and energy capacity, addressing the primary challenges associated with conventional liquid electrolytes. Moreover, the material's unique structure deviates from traditional solid electrolytes, broadening the chemical space for further discoveries and optimizations.
Battery Energy Triumph
The researcher’s solid-state breakthrough could enhance the performance and safety of lithium-ion batteries, with implications ranging from electric vehicles to portable electronics. Beyond this, the development paves the way for continued advancements in solid electrolyte research, offering the potential for even more efficient and sustainable energy storage solutions in the future.