Nano-Structuring Enables Next-Gen Energy Technologies
MIT researchers engineer nanoparticles with ion irradiation to create efficient catalysts and microelectronics.
Nanoparticles play a critical part in reactions, including fuel cells and electrolysis, by acting as catalysts or electrodes. For instance, platinum nanoparticles serve as efficient catalysts in water electrolysis. The size and composition to control can enhance their catalytic activity, selectivity, and durability.
Enlarged image of nanoparticles. Image used courtesy of Lawrence Berkeley National Laboratory
The unique properties of nanoparticles stem from their small size, which leads to a high surface area-to-volume ratio. This increased surface area exposes more active sites for chemical reactions, enhancing efficiency and reactivity. However, tailoring nanoparticles is difficult due to degradation over time and their sensitivity to the environment.
MIT researchers have demonstrated a way to tailor nanoparticle properties by leveraging ion irradiation, in which beams of charged particles are exposed to the material. They report that their developed nanoparticles have superior performance over conventionally created particles.
Applications of Nanoparticles and Challenges in Controlling Their Properties
Electrochemical processes like electrolysis and fuel cells involve an electric current passing through an electrolyte to decompose a compound into its constituent elements. In fuel cells, water molecules split into hydrogen and oxygen ions.
Nanoparticles offer several advantages for electrocatalysts in electrolysis. One main advantage is the nanoparticles facilitate the transfer of electrons between the electrolyte and the catalyst, speeding up the reaction. Also, nanoparticles have a large surface area, exposing more active sites for the reaction. However, metal catalysts usually used in electrolysis coarsen at high temperatures, losing surface area and reactivity. Therefore, scientists desire to create stable, durable nanoparticles insensitive to high temperatures.
Modifying nanoparticles is challenging because their properties are often interconnected, making it difficult to manipulate one without affecting the others. An additional issue is maintaining the stability of particles during synthesis, storage, and application. A previous solution involving exsolution by Jiayue Wang, a former MIT PhD student, now a postdoc at Stanford University, produces stable active nanoparticles. However, the particles are difficult to control.
Ion Irradiation for Nanoparticle Engineering
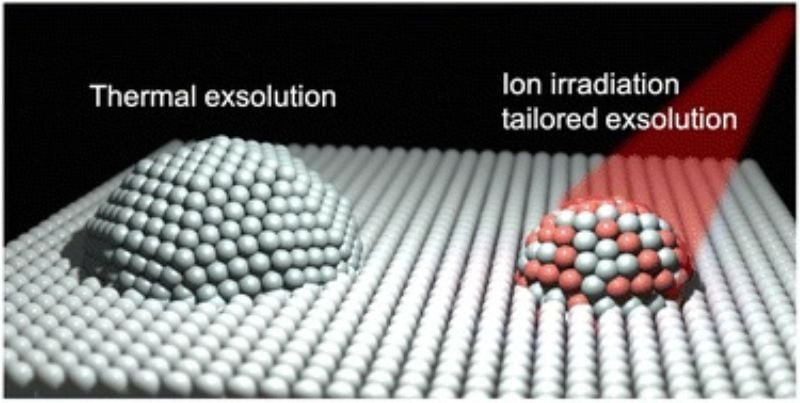
MIT researchers have now found a way to control the active nanoparticles by leveraging the ion irradiation technique. They discovered that aiming ions at the electrode while exsolving metal nanoparticles on the electrode's surface allows them to alter many properties of the particles.
Comparison between nanoparticles with exsolution and combining ion irradiation and exsolution. Image used courtesy of MIT
In ion irradiation, ions are accelerated at very high speeds to penetrate the material and cause changes in its structure and properties. The ion beam can knock atoms out of their lattice, forcing them to rearrange and change crystal structure. Similarly, this method can cause nanoparticles to shrink or grow in size. Moreover, it can also make them more stable by making them less susceptible to aggregation or degradation.
The researchers demonstrated this method by bombarding nickel ions on exsolved metal nanoparticles. Researchers state their methodology is substrate-oxide independent, allowing the freedom to select both oxide and ions for irradiation and exsolution. Wang, the lead author, believes their method has the potential to create well-controlled micro- and nano-structures for a variety of applications.